How to Find Experimental Molar Mass
Take a standard chemistry formula for a molecule split it up into the component atoms and look up the molar weight of each atom. Using atomic masses to calculate the molar mass.

1 3 Determine The Molar Mass Of A Gas Experimentally Youtube
Empirical formula weight 1 x 1201gmol 2 x 101gmol 1 x 1600gmol 3002gmol.

. Molar mass refers to the mass of a compound per unit mole. Element percentage mass in grams textm Step 2. The procedure to use the molar mass calculator is as follows.
Applications and skillsObtaining and using experimental values to calculate the molar mass of a gas from the ideal gas equation. Using the mass calculated in Calculation 7 and the number of moles calculated in Calculation 12 calculate the experimental molar mass of butane. In grams molar mass is numerically equal to.
Enter the molecular formula of the substance. Determine the molal concentration m from the change in boiling point and the boiling point elevation constant. Add the weight of the atoms in the molecule and you have the molar mass for the molecule.
Molar mass solute. Use the freeing point depression to calculate the molality of the solution. She has taught science courses.
The molar mass of a substance can be calculated using several methods. Helmenstine holds a PhD. Using the equation to calculate the molar mass.
Mass of flask foil cover condensed ethanol 8 15 В 10069731004973 1102 113 147 1128 12 290mL 290 m2. This means the percent error is -70. Molar Mass and Vapor Density Data Trial 1 Trial 2 P Barometric pressure atm A.
Why is it so high assuming there is no relevant human error causing it. Divide the molar mass for the molecular formula by the empirical formula mass. Molar mass of an element is given as below.
Find molar mass Step 1. Calculate the mass of each element in grams. What does experimental molar mass mean.
Multiply the atomic weight of each element with its number of atoms present in the compound. Butane has the molecular formula C 4 H 10. The best way to calculate molar mass of a compound is by counting the number of atoms of a type present in it and by.
Now click the button Calculate Molar Mass to get the result. Molar mass of an element Relative atomic mass x molar mass constant gmol Relative atomic mass is the mass of an atom relative to the mass of Carbon-12 atom and it has no units. To find the molar mass find the atomic mass of all the components of a chemical.
It will calculate the total mass along with the elemental composition and mass of each element in the compound. Add up all and assign unit as gramsmole. Calculating the molar mass from the boiling point elevation.
Count the number of moles of each type of atom that is present. Mass solute 387 g. Enter the chemical compound in the respective input field.
___6583_____ gmol MM 0079 g 00012 mol 6583 gmol 14. I did a lab on the collection of butane over water to calculate the molar mass. Therefore 6219 x 10-3 mol of NaOH are required.
Determine the moles of. In biomedical sciences and is a science writer educator and consultant. Then use the molality equation to calculate the moles of solute.
This can be done simply by the addition of molar masses of each atom present. It usually has the units gmol1 g m o l 1 and can also be referred to as the molecular mass or molecular weight. Fe Au Co Br C O N F.
Finally the molar mass of the chemical compound will. Calculate its theoretical molar mass based on this formula. Make use of the chemical formula to determine the number of atoms of each element in the compound.
Mol Acid mol Base 0006219mol. Rmmatomic massrm rmmolar quantity left rmM right. Thus you may assume that m_c m.
Make a data table in either Word or Excel containing the information below. You can either memorize it or find all of the atomic masses located on the periodic table of. Reaction is known the molar mass of the unknown acid can be calculated by modification of equation 3.
How many moles of acid are present initially. From the experimentally determined value of m and the mass of solute added you can determine the molar mass of the unknown solute. How to find Molar Mass.
Find molar mass of an element Find the molar mass of an element. Calculate diatomic elements Remember that seven elements hydrogen nitrogen oxygen fluorine chlorine. Mass of flaskfoil cover e B.
In one trial the measured molar mass was 20 gmol however the accepted value for butane is 5814 gmol. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. The moles of base titrant can be determined from the molarity of the base solution multiplied by the volume of titrant required to reach the equivalence point or moles base M.
Consider chemical compounds without. Determining Molar Mass Determine the change in boiling point from the observed boiling point of the solution and the boiling point of the pure. Use uppercase for the first character in the element and lowercase for the second character.
Then divide the grams of solute by the moles to determine the molar mass. Since only non-dissociating solutes will be used in this experiment the value of i for your unknown solute can be considered to be 1. This program determines the molecular mass of a substance.
Mass H 2 O 218 g 0218 kg.

Chemistry 101 Determining Molecular Formula From Experimental Data Youtube
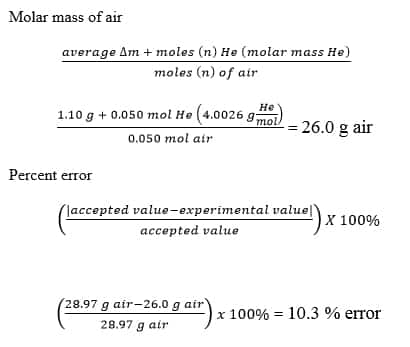
Molecular Weight Of Air Lab Explained Schoolworkhelper

Aleks Finding A Molecular Formula From Molar Mass And Elemental Analysis Youtube

How Muchm Molecular Mass Of Nacl Is Obtained Experimentally Using Colligative Properties Youtube
Comments
Post a Comment